Water Stress Management Webinar 28th March 2019 - Slides
These are the slides for the webinar on ways to assess and manage “Water Stress” inside your company and in your supply chain.
Access our full library of resources, register for events and webinars and share audits and other assessments
Our resource library is made available freely to all in order to advance responsible supply chain practices. Here you will find information and guidance on responsible supply chain issues and the way that the PSCI works. If you have any suggestions for improvements or additions to our resources please contact us.
This is a recording of the webinar on ways to assess and manage “Water Stress” inside your company and in your supply chain.
Please find a link to the webinar on the latest SAQ & Audit Report Template Update.
WORD VERSION
Abbreviated PSCI Self Assessment Questionnaire & Audit Report Template for Service Providers & General Manufacturers
Version 5, February 2019
Version 6, remote audit options added, October 2020
Since 20 April 2022, the Word versions of the templates (Full, Abbreviated, CAPR) have been removed completely following a consultation process and notice period. Access to these is only be possible in exceptional circumstances and by contacting the PSCI Secretariat. Please use the Excel versions: Full, Abbreviated, CAPR
This PSCI Audit Sharing Platform User Guide explains how to use the Audit Sharing Platform for members. The platform underpins the PSCI Audit Sharing Program. It is the platform for sharing audits with the PSCI membership.
Please refer to the PSCI Shared Audit Program Guidance for more information about the PSCI Audit Sharing Program.
This document is designed to be used by PSCI members, audit contractors and suppliers. It provides a detailed overview of the audit process and corresponding roles and responsibilities at each stage of the process.
Version 8, November 2025
Updated to align with the updated PSCI Principles and Strategy, inclusion of the digital Data Sharing via the PSCI Audit Platform, updated audit uploading requirements for full members, introduced online SAQ and removed reference of word version template, referenced the Audit Checklist as a separate resource in Chapter 10 and added section on Managing Audit Related Issues in the appendix.
This PSCI webinar is a training video for auditors. This was recorded in September 2018.
The slides shown in this webinar can be found here.
Day 2 presentation pack from the 2018 Supplier Conference in Shanghai, China.
Day 1 presentation pack from the 2018 Supplier Conference in Shanghai, China.
This is the agenda for 2018 PSCI India Supplier Conference.
This is recording of the PSCI sponsored webinar on evaluating supplier ethics and compliance practices and programs which took place on 25th July 2018.
This is the agenda for 2018 PSCI China Supplier Conference.
This is the slide deck from the PSCI sponsored webinar on how to manage APIs in manufacturing effluent (Part 4) which took place on 10th July 2018.
The webinar gave a brief refresher on Pharmaceuticals in the Environment (PiE), Anti-Microbial Resistance (AMR) and Predicted No-Effect Concentration (PNEC) first principles, and introduced a PSCI resource page where PNECs can be found. It also included step-by-step guidance on how to locate PNECs and use them.
The webinar recording is available here.
This is recording of the PSCI sponsored webinar on how to manage APIs in manufacturing effluent (Part 4) which took place on 10th July 2018.
The webinar gave a brief refresher on Pharmaceuticals in the Environment (PiE), Anti-Microbial Resistance (AMR) and Predicted No-Effect Concentration (PNEC) first principles, and introduced a PSCI resource page where PNECs can be found. It also included step-by-step guidance on how to locate PNECs and use them.
The webinar slide deck is available here.
This document defines the PSCI member audit uploading requirement.
Refreshed in May 2025 to align with the audit uploading expectations in the updated PSCI Strategy 2024 - 2026.
The following are useful resources for obtaining PNEC values.
Inside the report, you will find highlights and achievements from 2017, a busy and important year for PSCI, and our plans for 2018.
This is a short summary of the new strategy, and the process taken to achieve it.
Presentation Pack from the 2017 PSCI Auditor Training, 20 - 22 November, in Shanghai, China.
Presentation Pack from the 2017 PSCI Auditor Training, 20 - 22 November, in Shanghai, China.
Presentation Pack from the 2017 PSCI Auditor Training, 20 - 22 November, in Shanghai, China.
This Excel template is designed to help PSCI members, suppliers and audit firms check auditors against the PSCI's requirements, to ensure that they have the expertise needed to conduct PSCI audits. It is also used by new audit firms in their application process to demonstrate their capacity.
This evaluation tool is also known as PSCI Auditor Information Template (HSE & Social Auditors).
Last updated March 2021.
Agenda for 2017 Supplier Conference in Hyderabad, India
Green Chemistry Pharma Week Photo Report February 2017
Presentation booklet from the 2017 PSCI Auditor Training, 28 Feb - 1 March, in Hyderabad, India
This is the slide deck for the PSCI sponsored webinar on how to manage APIs in manufacturing effluent (Part 3) which took place on 25th October 2016. The webinar looked at advanced technologies to reduce API loss with guest speakers from the Temple University WET Centre and AECOM.
This is recording of the PSCI sponsored webinar on how to manage APIs in manufacturing effluent (Part 2) which took place on 15th June 2016. The webinar provided step-by-step guidance on this ‘spotlight’ issue for our industry and covered the following topics:
The webinar's slide-deck is available here.
This is the slide deck for the PSCI sponsored webinar on how to manage APIs in manufacturing effluent (Part 2) which took place on 15th June 2016. The webinar provided step-by-step guidance on this ‘spotlight’ issue for our industry and covered the following topics:
The webinar recording is available here.
This is the slide deck for the PSCI sponsored webinar on how to manage APIs in manufacturing effluent which took place on 27th January 2016. The webinar provided step-by-step guidance on this ‘spotlight’ issue for our industry and covered the following topics:
The webinar recording is available here.
This is a recording of the PSCI sponsored webinar on how to manage APIs in manufacturing effluent which took place on 27th January 2016. The webinar provided step-by-step guidance on this ‘spotlight’ issue for our industry and covered the following topics:
The webinar slide-deck is available here.
This PSCI Audit Sharing Platform User Guide explains how to use the Audit Sharing Platform for suppliers. The platform underpins the PSCI Audit Sharing Program. It is the platform for sharing audits with the PSCI membership.
Please refer to the PSCI Shared Audit Program Guidance for more information about the PSCI Audit Sharing Program.
Abstract: This Caldwell et al. study describes guidance intended to assist pharmaceutical manufacturers in assessing, mitigating, and managing the potential environmental impacts of active pharmaceutical ingredients (APIs) in wastewater from manufacturing operations, including those from external suppliers.
This document explains the PSCI membership fees structure and the Supplier Partnership fee.
New version added: October 2024
PowerPoint on the fundamentals of industrial hygiene.
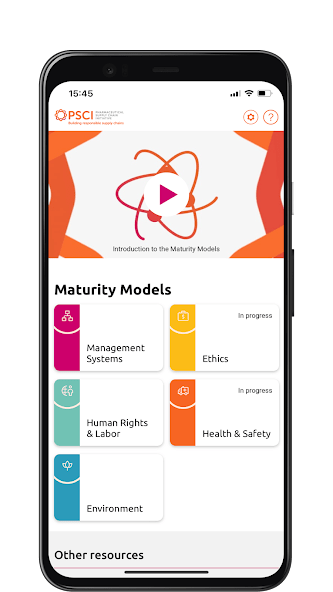
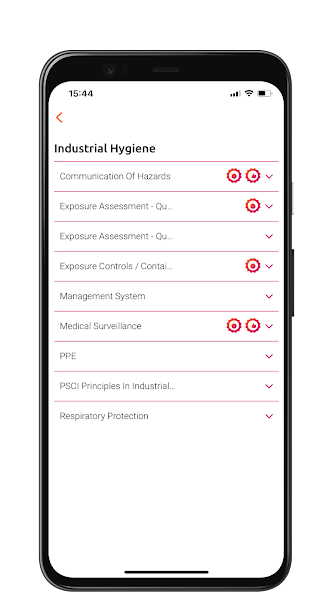
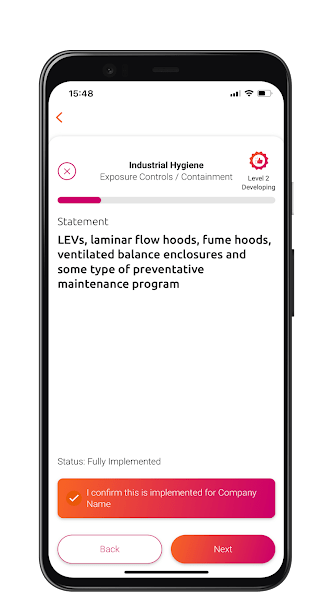
The PSCI Maturity App provides an interactive experience of the PSCI Maturity Models. Available on Android and iOS.