PSCI Supplier Newsletter January 2026
Get a quick update on the latest PSCI news. From the newly announced 2026 Board to the launch of the integrated online audit process, new learning courses, upcoming webinars, and more.
Access our full library of resources, register for events and webinars and share audits and other assessments
Our resource library is made available freely to all in order to advance responsible supply chain practices. Here you will find information and guidance on responsible supply chain issues and the way that the PSCI works. If you have any suggestions for improvements or additions to our resources please contact us.
Get a quick update on the latest PSCI news. From the newly announced 2026 Board to the launch of the integrated online audit process, new learning courses, upcoming webinars, and more.
About: This webinar is presented in Japanese.
Antimicrobial Resistance (AMR) is a critical global challenge that poses serious threats to healthcare systems worldwide. This webinar provides a clear overview of the fundamentals of AMR and outline how the pharmaceutical industry should respond to this pressing issue.
It introduces the Antibiotic Manufacturing Standard developed by the AMR Industry Alliance and highlight emerging certification schemes designed to ensure responsible practices across the industry. In addition, key initiatives led by the SHIONOGI Group will be presented, illustrating the path toward sustainable healthcare.
This session offers a valuable opportunity to deepen your understanding of AMR and explore practical approaches to addressing this global concern.
Speakers
The slides used in the webinar can be accessed here.
About : This webinar is presented in Mandarin. It decodes the “Three-Step Framework” of the decarbonisation handbook, using seven key impact areas in the pharmaceutical industry—R&D/Clinical, Laboratories, Production & Energy, Supply Chain & Logistics, Packaging, and Green IT—as a guide.
It breaks down the potential, cost, implementation timeline, and regulatory complexity of 24 actionable carbon reduction measures one by one, helping EHS, Procurement, and Operations teams collaboratively develop a localized priority action list. Additionally, it provides an overview of six key organizational capabilities to support implementation from pilot to scale.
Speakers
PSCI China Website link: Toward Net-Zero - Interpreting the PSCI Decarbonization Playbook
About: This webinar is presented in Mandarin and the presentation slides are bilingual in Mandarin/English.
This webinar outlines the market for renewable energy procurement in China and opportunities to progress renewable energy sourcing.
Speakers
PSCI China Website link: Renewable Energy Sourcing: Market and Practice
2025 was another year of impact for the PSCI, full of highlights that were made possible by the dedication and expertise of our Members and delivered benefits for Members, suppliers, and ultimately patients across the world.
From projects to partnerships and beyond, the PSCI is the place to be for collaboration across the sector, creating change, impact, and value.
Through the contributions of the Board, Committees, Topic Teams, and Regional Teams, we are shaping the supplier market and delivering our mission of building responsible supply chains.
Watch this video to hear more.
The third session of the 2025 PSCI China Supplier Conference took place on November 19, covering Environment, Decarbonisation, Audit.
2025年中国供应商大会第三天于上海线下举行,覆盖安全总览、过程安全、工业卫生议题。
Speaker:
Liang Hong | Engineering Manager | WSP
演讲嘉宾:
洪亮 | 工程经理 | 科进柏诚工程技术(北京)有限公司上海分公司
Topic: The design, operation and maintenance of isolator
议题:隔离器的设计、使用和维护
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The third session of the 2025 PSCI China Supplier Conference took place on November 19, covering Environment, Decarbonisation, Audit.
2025年中国供应商大会第三天于上海线下举行,覆盖安全总览、过程安全、工业卫生议题。
Speaker:
David Lv | Technical Director | BEEREE Safety Technologies (Hangzhou) Co., Ltd.
演讲嘉宾:
吕华军 | 技术总监 | 碧瑞安全技术(杭州)有限公司
Topic: Discussions on Differences Between Exposure Assessment and Airtightness Testing
议题:暴露评估与密闭性测试的差异分享
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The third session of the 2025 PSCI China Supplier Conference took place on November 19, covering Environment, Decarbonisation, Audit.
2025年中国供应商大会第三天于上海线下举行,覆盖安全总览、过程安全、工业卫生议题。
Speaker:
Jiawei Zhang | IH Service Leader | Zhanghan Consulting (Shanghai) Co., Ltd.
演讲嘉宾:
张佳维 | 工业卫生服务经理 | 章含管理咨询(上海)有限公司
Topic: Comprehensive Lifecycle Management of Chemicals and Hazards
议题:化学品及其危害因素的全流程管理
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The third session of the 2025 PSCI China Supplier Conference took place on November 19, covering Environment, Decarbonisation, Audit.
2025年中国供应商大会第三天于上海线下举行,覆盖安全总览、过程安全、工业卫生议题。
Speaker:
Lemon Wu | IDP Section Manager | TUV SUD
演讲嘉宾:
吴宙遨 | 防爆部门经理 | 南德意志大中华集团上海分公司
Topic: Overview of Dust Explosion Parameter Measurement and Dust Removal System Design
议题:制药行业粉尘爆炸参数的测定与除尘系统设计概论
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The third session of the 2025 PSCI China Supplier Conference took place on November 19, covering Environment, Decarbonisation, Audit.
2025年中国供应商大会第三天于上海线下举行,覆盖安全总览、过程安全、工业卫生议题。
Speaker:
Roderick Yuan | Technical Director | Shanghai STRM Technology Co., Ltd.
演讲嘉宾:
员文权 | 技术总监 | 上海翊员科技有限公司
Topic: Application of LOPA in SIL Determination
议题:LOPA在SIL定级中的应用
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The third session of the 2025 PSCI China Supplier Conference took place on November 19, covering Environment, Decarbonisation, Audit.
2025年中国供应商大会第三天于上海线下举行,覆盖安全总览、过程安全、工业卫生议题。
Speaker:
Ken Sun | AP EHS&S Lead | GSK
演讲嘉宾:
孙大勇 | 亚太区EHS与可持续发展经理 | 葛兰素史克
Topic: PSCI Audit Findings on Process Safety and Incident Sharing
议题:安全事故案例分享及PSCI过程安全相关审计发现
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The second session of the 2025 PSCI China Supplier Conference took place on November 18, covering Environment, Decarbonisation, Audit.
2025年中国供应商大会第二天于上海线下举行,覆盖环境、节能减排、审计议题。
Speaker:
Lei Jiang | EHS Executive Director | Pharmaron Shaoxing Co., Ltd.
演讲嘉宾:
蒋磊 | EHS执行总监 | 康龙化成(绍兴)药业有限公司
Topic: Environmental practice based on the PSCI Management Maturity Model
议题:基于PSCI成熟度模型的环境管理实践
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The second session of the 2025 PSCI China Supplier Conference took place on November 18, covering Environment, Decarbonisation, Audit.
2025年中国供应商大会第二天于上海线下举行,覆盖环境、节能减排、审计议题。
Speaker:
Jinyun Tang | EHS Lead | Chongqing Carelife Pharmaceutical Co., Ltd.
Wenjun Wang | Senior EHS Manager | Pfizer
Victor Ren | Supplier HSE Assurance Manager | Sandoz
演讲嘉宾:
唐金云 | EHS主管 | 重庆凯林制药有限公司
王文君 | 高级EHS经理 | 辉瑞
任维农 | 供应商HSE保障主管 | 山德士
Topic: Round Table Session: Practice Sharing for AMR Certification
议题:圆桌讨论:AMR认证实践经验分享
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The second session of the 2025 PSCI China Supplier Conference took place on November 18, covering Environment, Decarbonisation, Audit.
2025年中国供应商大会第二天于上海线下举行,覆盖环境、节能减排、审计议题。
Speaker:
Thea Zhang, EHS Supervisor, Boehringer-Ingelheim
演讲嘉宾:
张前雯,EHS主管,勃林格殷格翰
Topic: PSCI Supplier Decarbonisation Playbook
议题:PSCI供应商减碳手册解读
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The second session of the 2025 PSCI China Supplier Conference took place on November 18, covering Environment, Decarbonisation, Audit.
2025年中国供应商大会第二天于上海线下举行,覆盖环境、节能减排、审计议题。
Speaker:
David Wei | Associate Director of Utility Management | AstraZeneca
Shuji Chen | Associate Director | EECO2 China (affiliated with Energy Efficiency Consultancy Group Limited)
演讲嘉宾:
韦雁翔 | 介质管理副总监 | 阿斯利康
陈蜀冀 | 副总监 | 易客图(苏州)信息有限公司
Topic: Improving Energy Efficiency
议题:能源效率提升的实施路径
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2025 PSCI China Supplier Conference took place on November 17, covering Introduction, Management System, Human Rights & Ethics.
2025年中国供应商大会第一天于上海线下举行,覆盖PSCI 最新发展、管理体系、劳工与商业道德议题。
Speaker:
Minnie Mai | Senior Technical Manager | TUV Rheinland
演讲嘉宾:
麦璐 | 高级技术经理 | TUV莱茵
Topic: Information Security & Personal Data Protection in PSCI Audits/AI Application
议题:PSCI审计/AI应用中的信息安全与隐私数据保护
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2025 PSCI China Supplier Conference took place on November 17, covering Introduction, Management System, Human Rights & Ethics.
2025年中国供应商大会第一天于上海线下举行,覆盖PSCI 最新发展、管理体系、劳工与商业道德议题。
Speaker:
Xiaoming Wang | Principal Consultant | QD Wagner Biotechnology Consulting Co., Ltd.
演讲嘉宾:
王晓明 | 首席顾问 | QD瓦格纳生物科技咨询有限公司
Topic: Bacteria Endotoxin Testing Theory & Practice
议题:细菌内毒素检测的理论与实践
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2025 PSCI China Supplier Conference took place on November 17, covering Introduction, Management System, Human Rights & Ethics.
2025年中国供应商大会第一天于上海线下举行,覆盖PSCI 最新发展、管理体系、劳工与商业道德议题。
Speaker:
Lena Pan | Team Leader | Baohua Law Firm
演讲嘉宾:
潘丽娜 | 团队负责人 | 保华律师事务所
Topic: Occupational Health & Safety Management and Social Insurance
议题:劳动用工合规——职业健康安全与社会保险制度
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2025 PSCI China Supplier Conference took place on November 17, covering Introduction, Management System, Human Rights & Ethics.
2025年中国供应商大会第一天于上海线下举行,覆盖PSCI 最新发展、管理体系、劳工与商业道德议题。
Speaker:
Emma Lu | Technical Solution Manager | BSI
演讲嘉宾:
卢峻仪 | 技术方案经理 | 英国标准协会
Topic: How to ensure working hours and salary benefits‘ compliance
议题:如何确保员工工作时间和薪资福利符合要求
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2025 PSCI China Supplier Conference took place on November 17, covering Introduction, Management System, Human Rights & Ethics.
2025年中国供应商大会第一天于上海线下举行,覆盖PSCI 最新发展、管理体系、劳工与商业道德议题。
Speaker:
Felix Ding | Region HSE Lead | Novartis
Graham Ding | HSE EPRM Business Partner | Novartis
演讲嘉宾:
丁晓阳 | 地区HSE主管 | 诺华
丁周琛 | HSE EPRM 业务伙伴 | 诺华
Topic: Building an ESG Management Structure
议题:ESG管理体系搭建
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2025 PSCI China Supplier Conference took place on November 17, covering Introduction, Management System, Human Rights & Ethics.
2025年中国供应商大会第一天于上海线下举行,覆盖PSCI 最新发展、管理体系、劳工与商业道德议题。
Speaker:
Li Liu | EHS&S Manager| Boehringer-Ingelheim / PSCI China Regional Committee Co-Lead
演讲嘉宾:
刘立 | EHS&S 主管 | 勃林格殷格翰 / PSCI中国区负责人
Topic: Updates on PSCI
议题:PSCI最新动态
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2025 PSCI China Supplier Conference took place on November 17, covering Introduction, Management System, Human Rights & Ethics.
2025年中国供应商大会第一天于上海线下举行,覆盖PSCI 最新发展、管理体系、劳工与商业道德议题。
Speaker:
Fei Ren | Director of DIvision III of Multilateral Cooperation, Department of Multilateral Cooperation | Deputy Secretary General of Sustainable Markets Initiative China Council
演讲嘉宾:
任飞 | 中国国际商会多边部多边合作三处处长 | ”可持续市场倡议“中国理事会副秘书长
Topic: Deepen Green Transformation and Jointly Build a Zero-Carbon Healthcare System —— Practice and Outlook of the Health Working Group under the Sustainable Markets Initiative China Council
议题:深耕绿色转型,共筑零碳医疗 ——“可持续市场倡议” 中国理事会健康系统工作组实践与展望
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The pharmaceutical sector's Scope 3 emissions from purchased goods and services total over 272 million tCO2-e, representing more than 80% of the industry's carbon footprint. This webinar highlights how suppliers across the pharmaceutical value chain are driving verified, measurable climate impact and helping companies meet rising ESG expectations.
Join James Connelly, CEO of My Green Lab, to deep dive into the tools empowering pharmaceutical suppliers to deliver verified sustainability performance. He will cover the Converge Supplier Initiative, My Green Lab Certification, the ACT RFP Letter, ACT API integration into purchasing platforms, and the ACT Ecolabel for verified claims. James will also share insights from My Green Lab's 2025 Carbon Impact Report, showing how supplier engagement accelerates Scope 3 reductions. Whether you’re a supplier seeking to differentiate or a pharmaceutical company aiming to strengthen supplier collaboration, this session offers actionable pathways to achieve measurable climate results.
The associated slides for this recording can be found here
Pharmaceutical and healthcare companies run thousands of audits of their suppliers’ sites annually. Audits typically require between two to four days of focused, on-site effort from both the auditor and the supplier. PSCI members work together to share these audits, in support of greater transparency and valuable efficiencies that benefit both members and suppliers.
This report shares our analysis of findings from PSCI audits available on our platform. Each year, we review these in line with the PSCI Principles to identify trends, highlight improvements, and share recommendations. PSCI work with member companies, suppliers, and regional partners to turn analysis into practical guidance, training, and peer learning that suppliers can implement quickly. Overall, this analysis provides deep insight into supplier practices within the pharmaceutical and healthcare industry and suggests where collaborative efforts will have the greatest impact, guiding us on developing targeted resources and trainings.
This year’s report analyzes 2,046 findings from 228 supplier audits uploaded to the PSCI platform in 2024. These findings are categorised into 428 Governance & Management Systems findings, 26 Ethics findings, 273 Human Rights findings, 1,098 Health & Safety findings, and 221 Environment findings, offering a comprehensive overview of supplier practices and opportunities for improvement. The report includes a summary of findings across key topics, as well as a regional analysis looking at patterns across China, India, US, Western Europe, and other regions represented in this year’s uploads, presenting recommended actions for suppliers.
The commercial and sales teams in the pharmaceutical industry spend a significant amount of time traveling to meet healthcare providers, distributors, and customers across diverse geographies. Road safety is the number one risk in India and road incidents directly impact personal safety, business continuity, and customer engagement.
This session will cover key aspects of road safety including safe driving practices and behaviours, managing fatigue and stress on the road, and the importance of avoiding distractions while driving. Demonstrating leadership in road safety strengthens employee engagement, corporate reputation, supports ESG commitments, and contributes to India’s National Road Safety Mission. By participating, you can help your organizations drive collective action towards a safer, more responsible, and resilient pharmaceutical ecosystem.
Speakers:
Mahesh Chandak
Head of SSHE - South Asia and Global Road Safety Lead, Bayer
Rajani Chavan
SSHE Enablement Lead, South Asia and India Road Safety lead, Bayer
The associated slides can be downloaded here
The commercial and sales teams in the pharmaceutical industry spend a significant amount of time traveling to meet healthcare providers, distributors, and customers across diverse geographies. Road safety is the number one risk in India and road incidents directly impact personal safety, business continuity, and customer engagement.
This session will cover key aspects of road safety including safe driving practices and behaviours, managing fatigue and stress on the road, and the importance of avoiding distractions while driving. Demonstrating leadership in road safety strengthens employee engagement, corporate reputation, supports ESG commitments, and contributes to India’s National Road Safety Mission. By participating, you can help your organizations drive collective action towards a safer, more responsible, and resilient pharmaceutical ecosystem.
Speakers:
Mahesh Chandak
Head of SSHE - South Asia and Global Road Safety Lead, Bayer
Rajani Chavan
SSHE Enablement Lead, South Asia and India Road Safety lead, Bayer
The recording for this webinar can be accessed here
The PSCI was delighted to exhibit at CPHI Frankfurt 2025 as an Official Sustainability Partner of the conference. We engaged with Members, Supplier Partners, and other Partners all working towards building responsible supply chains.
Check out our highlights video and see you next year at CPHI Milan!
The 2023 revision of the PSCI Principles (PSCI Principles v3.0) was approved by the membership on Tuesday 23rd May 2023, at the Spring Meeting in Paris (France), and became effective immediately.
The PSCI Principles for Responsible Supply Chain Management are foundational, setting members' expectations of suppliers, and form the base for all PSCI's tools and materials.
These Principles set the standard for governance and management systems, ethics, human rights, health and safety, and environmental business practices.
The Principles are adopted by all PSCI members and may be voluntarily supported by any business in the pharmaceutical and healthcare industry.
The countdown to the 2025 PSCI India Conference & Exhibition 2025 has begun, and the excitement is building!
The PSCI India team is thrilled to welcome all participants, speakers, presenters, and exhibitors for a dynamic event focused on building resilient supply chains through collaborative leadership.
The agenda is now live with sessions related to the PSCI Principles such as:
Download the agenda below
Join us for:
If you haven’t registered yet, now’s the time—secure your spot and be part of this impactful experience.
Please find the agenda for the PSCI Decarbonisation Summit 2025.
this agenda is subject to minor changes.
In the complex and highly regulated pharmaceutical and healthcare sectors, the ability to anticipate, withstand, respond to, and recover from disruptions is critical. This webinar will explore the fundamentals and strategic value of Business Continuity Management (BCM), with a particular focus on its alignment with supply chain risk management.
Attendees will gain insights into how BCM can be effectively integrated with incident and risk management systems to build resilience across operations. We’ll explore best practices for implementing a holistic, coordinated approach that ensures continuity in the face of global supply chain volatility, regulatory pressures, and emerging threats such as cyberattacks and natural disasters.
Why You Should Attend:
There will also be an opportunity to ask questions to our speaker during a dedicated Q&A session.
Speaker: Guy Stone
Head of Business Continuity Management for Roche Diagnostics since January 2025, responsible for defining and implementing the BCM Strategy for the Diagnostic Division of Roche and for providing central coordination as well as setting objectives and key results.
Associated recording available here
This one pager has been developed to provide a quick overview of the benefits of PSCI for suppliers to PSCI members.
We recommend that you share this with your suppliers whenever you are trying to provide a quick overview of PSCI & the benefits of engagement.
The PSCI Anti-trust Statement in English and Chinese.
2024 PSCI Chair Deirdre O’Reilly (Viatris) and 2024 Vice Chair Rob Williams (AstraZeneca) reflect on member-driven progress, shared achievements, and what’s next for the PSCI community. From deeper supplier engagement to an upgraded digital platform, hear how we’re continuing to build responsible pharmaceutical supply chains together.
An introduction deck for EQMS Ingenuity Private Limited
The PSCI presents its report on the use of Horseshoe Crab Blood for Endotoxin Testing in the Pharmaceutical Industry. The report provides insights into the context and complexities behind its use, pathways for better practices in the industry, an insight into the PSCI member companies’ use and next steps both the PSCI and others working in this ecosystem can take going forward.
The PSCI have been engaged on the topic of Horseshoe Crab Blood since 2023. Following the development of its position paper and convening through the working group. The PSCI has been discussing and exploring better pathways for better practices in the pharmaceutical industry and this report encouraging companies to minimize its use and seek alternatives for endotoxin testing in their supply chains.
Pharmaceutical companies use horseshoe crab blood to test medicines and vaccines for safety. The blood is a bright blue colour and has a special quality that makes it incredibly important to human health – it coagulates when exposed to bacterial endotoxins, which can cause sickness or even death in humans.
This project was funded through the projects workstream. Following report publication, the working group aims to publish further capability building resources to support good practices amongst PSCI members. While members are progressing on developing positions on the reduction or elimination of TAL and LAL in their supply chains, only few are be planning for the resilience of its supply.
The PSCI would like to extend a big thank you to Jay Bolden and Shah Shaid, members of the Horseshoe Crab Blood Working Group, the PSCI member companies that responded to the survey and all other contributors for the development of this year’s report.
The PSCI has launched our Supplier Partnership program to formally recognise and acknowledge suppliers’ commitment to responsible business practices. Becoming a PSCI Supplier Partner means committing to the PSCI Principles and contributing to a community committed to driving responsible value chains.
This presentation summarises the supplier partnership and provides an overview including:
The SAQ/Audit & Audit Guidance sub-team are pleased to publish the PSCI Pre-Audit Guidance Document Checklist. The checklist has been published as a separate document, previously found in the Audit Guidance as Annex 1.
The audit checklist summarizes important documents which the audit team may want to see in advance for audit preparation or want to review during the onsite audit visit. Depending on the type of supplier or the information provided as per SAQ, the list may be shortened (e.g. for service providers) or extended (e.g. for complex chemical or pharmaceutical manufacturers).
The checklist has been drafted using the current Full SAQ and Audit Report Template, suggesting documents that may be required for each principle (Governance & Management Systems, Ethics, Human Rights, Environment and Health & Safety) and corresponding question.
Climate change poses the greatest health threat of the 21st century and the pharmaceutical industry is a key contributor to global emissions due to complex product lifecycles and an energy-intensive value chain.
Many pharmaceutical companies have set ambitious, science-based decarbonization goals but only 20% of life sciences companies are on track to achieve net zero by 2050. Advancing decarbonization can be slow as companies wrestle with various pain points across the product lifecycle but delays in decarbonizing expose companies to risks across the stakeholder landscape. Decarbonizing the pharmaceutical industry is a core component of the PSCI’s Environment Principle and PSCI members realize the importance of working together and with their suppliers across the whole value chain.
There have been several whitepapers published on the topic of health sector decarbonization. This Playbook builds on that work to provide 24 detailed emissions reduction initiatives across 7 impact areas that map to the drug development lifecycle. The initiatives were assessed in terms of addressability, emission reduction potential, implementation timeline, upfront cost, and regulatory complexity, and intervention adoption timeframe.
We welcome your feedback. If you have any input on the Playbook, please contact us for consideration in future updates.
Revised January 2025
EXCEL VERSION WITH IMPORT SHEET (FOR ONLINE SAQ ONLY)
PSCI Self Assessment Questionnaire and Audit Report Template for Service Providers & General Manufacturers with import sheet.
For optimum functionality, we recommend using the most up to date version Microsoft Excel. If you only have access to Excel 2003 or earlier, we recommend using the Word version of the SAQ.
To access the offline abbreviated SAQ template and full version history of the SAQ, follow this link.
Content version 8.0, simplified the Categorisation of Suppliers (Category A, B or C), removed/ added a few questions, rewording some questions, December 2021. Import sheet incorporated July 2023.
Content version 9.0, full review and update of the SAQ. Section names and all questions revised to reflect the updated PSCI Principles, September 2024. Import sheet incorporated December 2024.
Full version template for Core Suppliers, External Manufacturers, Component and Material Suppliers are also available and come in multiple languages: Excel version full.
Please note: Content version 9.0 is only currently available in English with translations to follow soon.
The previous version of the SAQ is currently available in the following languages:
The following links provide SAQ Online instruction documents for:
THE PSCI PRESENTS ITS SECOND PUBLIC AUDIT FINDINGS REPORT, PROVIDING CONTINUED INSIGHTS INTO SUPPLIER RISKS, TRENDS, AND AREAS FOR IMPROVEMENT ACROSS THE PHARMACEUTICAL AND HEALTHCARE SUPPLY CHAIN.
This report shares our analysis of findings from PSCI audits available on our platform. Each year, we review these in line with the PSCI Principles to identify trends, highlight improvements, and share recommendations. Overall, this analysis provides deep insight into supplier practices within the pharmaceutical and healthcare industry and guides us on developing targeted resources and trainings.
The year’s report analyzes 1,776 findings from 180 supplier audits uploaded to the PSCI platform in 2023. These findings are categorized into 308 Management Systems findings, 7 Ethics findings, 169 Human Rights findings, 970 Health & Safety findings, and 322 Environment findings, offering a comprehensive overview of supplier practices and opportunities for improvement. The report includes a summary of findings across key topics, detailed breakdowns with recommended actions for suppliers, and links to relevant
What’s new this year?
The report reinforces the PSCI’s role as a catalyst for continuous improvement, equipping suppliers with tools and guidance to enhance practices and adapt to evolving demands. For more details, access the full report in the resource attached.
You're Invited: PSCI Global Supplier Conference 2025!
Get ready for a highly regarded line-up of expert speakers, insightful discussions, and actionable strategies to drive sustainability in pharmaceutical supply chains.
🔎 Featured Sessions:
📅 Download the full agenda below. To register, click here.
Don’t miss this opportunity to connect, learn, and contribute to the future of sustainable value chains. Secure your spot today and make every session count!
📩 Spread the Word: Forward this invitation to your colleagues.
If you have any questions, feel free to reach out at info@pscinitiative.org.
See you there!
The first session of the 2024 PSCI China Supplier Conference took place on September 10, covering Management, Human rights & labor, and Decarbonization.
2024年中国供应商大会第一天于成都线下举行,覆盖管理体系、劳工、减碳议题。
Speaker:
Jennifer Jiang | Partner | ERM
演讲嘉宾:
姜煜瑶 | 合伙人 | 伊尔姆
Topic: Scope 3 and Decarbonization
议题:范围三和脱碳
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2024 PSCI China Supplier Conference took place on September 10, covering Management, Human rights & labor, and Decarbonization.
2024年中国供应商大会第一天于成都线下举行,覆盖管理体系、劳工、减碳议题。
Speaker:
Tracy Zhou | External Supply Chain EHS Manager | J&J
演讲嘉宾:
周穗菁 | 外部供应EHS经理 | 强生
Topic: The Management Maturity Model and Audit Findings
议题:PSCI治理与管理体系成熟度模型和审计发现项
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2024 PSCI China Supplier Conference took place on September 10, covering Management, Human rights & labor, and Decarbonization.
2024年中国供应商大会第一天于成都线下举行,覆盖管理体系、劳工、减碳议题。
Speaker:
Lin Wang, Director, BSR China
演讲嘉宾:
王林,中国区总监,商业社会责任
Topic: China labor market practice and impact to business
议题:中国劳动市场的实践以及对商业的影响
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2024 PSCI China Supplier Conference took place on September 10, covering Management, Human rights & labor, and Decarbonization.
2024年中国供应商大会第一天于成都线下举行,覆盖管理体系、劳工、减碳议题。
Speaker:
Jason Jiang| EHS Lead| GSK
Eric Peng| Regional Category Manager| Astra Zeneca
演讲嘉宾:
姜兴| EHS负责人 | 葛兰素史克
彭诗达| 亚太区采购经理 | 阿斯利康
Topic: Human Rights Audits Common Findings & CAPs
议题:劳动者权益审计常见发现项&整改方案| 劳动者权益话题分享
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2024 PSCI China Supplier Conference took place on September 10, covering Management, Human rights & labor, and Decarbonization.
2024年中国供应商大会第一天于成都线下举行,覆盖管理体系、劳工、减碳议题。
Speaker:
Jing DONG | Managing Director | Peterson Solutions China
演讲嘉宾:
董菁 | 总经理 | 上海佩笙检测有限公司
Topic: Social Sustainability in Upstream Supply Chain via Responsible Sourcing Process
议题:通过负责任采购实现上游供应链中的社会可持续发展
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2024 PSCI China Supplier Conference took place on September 10, covering Management, Human rights & labor, and Decarbonization.
2024年中国供应商大会第一天于成都线下举行,覆盖管理体系、劳工、减碳议题。
Speaker:
Gary Lu | APAC SHE & Sustainability Director | AstraZeneca
演讲嘉宾:
卢罡 | 亚太区安全健康环保及可持续发展总监 | 阿斯利康
Topic: China Green Power Project
议题:中国绿电项目
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2024 PSCI China Supplier Conference took place on September 10, covering Management, Human rights & labor, and Decarbonization.
2024年中国供应商大会第一天于成都线下举行,覆盖管理体系、劳工、减碳议题。
Speaker:
Joyce Jiang| Sr Sustainability & Compliance manager, Global Procurement| Roche (Shanghai) Pharmaceuticals Consulting Co.,Ltd.
演讲嘉宾:
蒋南婧 | 全球采购-可持续发展与合规高级经理 | 罗氏(上海)医药咨询有限公司
Topic: Roche’s Journey to Decarbonise its Supply Chain - Challenges & Mitigation
议题:罗氏供应链的减碳之旅-挑战和行动
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The second session of the 2024 PSCI China Supplier Conference took place on September 11, covering Environment, Industrial Hygiene, General Safety, and Process Safety.
2024年中国供应商大会第二天于南京线下举行,覆盖环境、工业卫生、安全总览、过程安全议题。
Speaker:
Don Yu| BD Manager| BSI
演讲嘉宾:
于东 | 商务经理 | 英国标准协会
Topic: BSI Kitemark™ for minimized risk of antimicrobial resistance
议题:BSI与抗生素耐药性认证
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The second session of the 2024 PSCI China Supplier Conference took place on September 11, covering Environment, Industrial Hygiene, General Safety, and Process Safety.
2024年中国供应商大会第二天于南京线下举行,覆盖环境、工业卫生、安全总览、过程安全议题。
Speaker:
Xiaoyuan Zhang| EHS Technical Generalist | Jiuzhou Pharmaceutical Co.LTD
演讲嘉宾:
张孝远| EHS技术总工 | 浙江九洲药业股份有限公司
Topic: Jiuzhou Pharmaceutical Process Safety Management Case Sharing
议题:九洲药业过程安全管理案例分享
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The second session of the 2024 PSCI China Supplier Conference took place on September 11, covering Environment, Industrial Hygiene, General Safety, and Process Safety.
2024年中国供应商大会第二天于南京线下举行,覆盖环境、工业卫生、安全总览、过程安全议题。
Speaker:
William Zhu | Associate Director | WSP
演讲嘉宾:
朱人 | 副技术总监 | 科进
Topic: Statistical Analysis of Industrial Hygiene Monitoring Data
议题:工业卫生数据的统计分析
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The second session of the 2024 PSCI China Supplier Conference took place on September 11, covering Environment, Industrial Hygiene, General Safety, and Process Safety.
2024年中国供应商大会第二天于南京线下举行,覆盖环境、工业卫生、安全总览、过程安全议题。
Speaker:
Roderick Yuan| Technical Director | Shanghai STRM Technology Co., Ltd.
演讲嘉宾:
员文权| 技术总监 | 上海翊员科技有限公司
Topic: Mechanical integrity: Safety critical equipment that are of concern – and how we maintain those
议题:设备完好性-关键安全设施及检查、检测和预防性维护
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The first session of the 2024 PSCI China Supplier Conference took place on September 11, covering Environment, Industrial Hygiene, General Safety, and Process Safety.
2024年中国供应商大会第二天于南京线下举行,覆盖环境、工业卫生、安全总览、过程安全议题。
Speaker:
Carl Deng | Senior Process Safety Consultant | DEKRA Shanghai Office
演讲嘉宾:
邓胤 | 高级过程安全顾问 | DEKRA 德凯达管理咨询(上海)有限公司
Topic: Mechanical Equipment Ignition Risk Assessment (MEIRA)
议题:机械设备点火风险评估(MEIRA)
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮
The 2024 PSCI India Supplier Conference in Goa brought together over 700 participants for three days of insightful discussions and networking. Featuring keynote speeches from industry leaders, expert panels, PSCI’s first-ever exhibition, and an auditors' panel, the conference showcased the PSCI’s commitment to advancing responsible supply chain practices.
Thank you to everyone who joined us!
Please find the 2024 China supplier conference video recap for event highlights.
Thanks everyone for joining us!
This slide deck, presented by N T Prasad, Head EHSS Bangalore, Syngene International Limited during the "Process Safety" session at the PSCI India Supplier Conference 2024 in Goa, India.
This resource covers key trends, challenges, and best practices relevant to PSCI members and suppliers, the resource is designed to enhance understanding, with actionable strategies and case studies relevant to India and the broader region. Perfect for those looking to deepen their knowledge in sustainable supply chain management and industry collaboration.
During 2024 we will be updating/replacing The Link – the online platform by which we communicate with members and share audits.
We have today launched an open tender exercise to find the best provider. The attached documents have been sent to a small group of selected bidders.
We would welcome the widest possible range of submissions.
Supporting documents:
PSCI Link 2.0 RFP - via the download button below.
Link system map
Link 2.0 user requirements
For ease, please note the following:
Address for correspondence is: LinkRFP@pscinitiative.org
Please confirm your interest to participate by: 10th May 2024
Bid submission deadline: 31st May 2024
PSCI has conducted a materiality review for the sector every few years (2013, 2017, 2020). We are pleased to publish our latest assessment, this time applying a double materiality framework. The assessment is specifically focused on supply chains for the Pharmaceutical & Healthcare industries, and takes into account member views and a range of outside sources. (For more detail about the double materiality approach, please see here.)
The initial materiality was conducted in Summer 2023 to support development of the new strategy. This has since been updated to take into take into account the final CSRD and to add an additional checking stage to the scoring and issues list.
We encourage members, suppliers and our other stakeholders and invited to read the report and use it as an input to their own planning processes.
For anyone with comments to the report, please contact Rosie.Towe@Carnstone.com.
As part of the PSCI's goal to promote consistency, quality, and innovation in all aspects of supplier evaluation and audit, we analyze PSCI audits conducted and uploaded on our shared platform on an annual basis. This report shows what we’ve found by analyzing 3,500 findings from 339 PSCI audits conducted during 2020-2022 and uploaded to the PSCI platform by the end of 2022.
We are specifically interested in understanding how the findings are distributed across the five areas of the PSCI Principles, our blueprint for responsible supply chain management & foundation of our tools. This analysis gives us visibility over how findings are changing over time and our impact on the industry.
This report, the first we have shared publicly, shows the results of a deep dive analysis into each area, focusing on 1,955 findings from 195 PSCI audits uploaded by the end of 2022. These findings reveal key risks, areas for improvement, and opportunities for PSCI members to support their suppliers to improve their practices.
This analysis also provides a high-level understanding of regional differences and levels of maturity, allowing us to provide regionally specific support on hot topics through our capability building work and begin to understand our own impact on the industry.
Speaking of the report's publication, Dorota Wiacek-Trojanowska, Audit Committee member says,
The annual analysis of audit results carried out by the PSCI not only provides information enabling the identification of the most important challenges faced by both PSCI members and suppliers, but also shows new challenges that we must be able to face. The audit results give us the opportunity to collect and analyze information on the biggest challenges in ensuring a sustainable supply chain in the whole pharma sector.
Please find the India Supplier Conference 2023 Video Recap for highlights of the event. Thanks to everyone who joined us for this exciting event!
Bylaws of the Pharmaceutical Supply Chain Initiative. Last updated in January 2024.
The PSCI has published our 2022 Annual Report, summarizing a year of growing connections and clear movement towards achieving our shared vision of excellence in safety, environmental, and social outcomes across the global pharmaceutical and healthcare supply chain.
Key developments in 2022 include
Vicki Stone-Bjarup, 2022 Chair of the PSCI, says,
Sectors around the world are working to tackle the sustainability challenges of their industry. This report transparently shares the successes that PSCI members have achieved for our sector over the last year, as well as the journey left to take. There is still a long way to go, but we are so proud to be a part of the progress being made in the pharmaceutical industry. Our focus remains to support our members to build responsible supply chains.
Read about these and more in the report below.
Presentation given by Balaji G. at the Musi River Wastewater Workshop 2023.
Decarbonizing the pharmaceutical industry in line with global efforts is a core component of PSCI’s environmental principle. Action is needed and PSCI members realize the importance of working together with their suppliers, often the majority of the footprint across the whole value chain.
This is why we're pleased to launch the PSCI Decarbonization Pathway, a tool that the industry can use to move towards a net zero value chain, in line with global goals such as the COP Paris Agreement and UN Sustainable Development Goals. The maturity model provides a clear pathway for suppliers to develop their capability and response towards reducing their carbon emissions.
PSCI Members, through their Decarbonization team and partnerships with leading organisations, will continue to provide the resources that suppliers will need in this journey.
Version: v3 August 2025
In this video, the PSCI reiterates its commitment to supporting our members to understand human rights practices in pharmaceutical supply chains and outlines the resources available to help make it happen across the industry.
Together with our members, the PSCI understands that society and business are best served by responsible business behaviors and practices. With our training materials, conferences, and tools, we aim not only to ensure the upholding of our Principles, but also to support all our members in implementing the United Nations’ Guiding Principles on Business and Human Rights in their supply chain and improving their human rights impacts around the world.
The first session of the 2022 PSCI China supplier conferences took place virtually on 6 September and covered Ethics, Labor, Management systems.
2022年中国供应商线上会议第一场于9月6日在线上举行,覆盖伦理,劳工,管理议题。
Speaker: Roderick | Technical director | Shanghai STRM Technology Co., Ltd.
演讲嘉宾:员文权 | 技术总监 | 上海翊员科技有限公司
Topic: Business continuity plan
议题:业务连续性计划
Watch the recording here
点击此处查看回放
To access the slides, please click "DOWNLOAD". 下载会议PPT,请点击下方DOWNLOAD按钮·
The third session of the 2022 PSCI India supplier conferences took place in-person on the 23rd of September and covered Human Rights & Labor and Ethics.
The full slide deck is available for download below.
The presentations covered are as follows:
The second session of the 2022 PSCI India supplier conferences took place in-person on the 22nd of September and covered Audit, Environment, Scope 3, PiE/AMR
The full slide deck is available for download below.
The presentations covered are as follows:
The first session of the 2022 PSCI India supplier conferences took place in-person on the 21st of September and covered Safety and Process Safety Management.
The full slide deck is available for download below at "View More".
The presentations covered are as follows:
The third session of the 2022 PSCI China supplier conferences took place virtually on 8 September and covered PiE / AMR and Environment.
Please click “DOWNLOAD” to access to session 3's deck.
The third session includes:
Management of change
Genping Zou | APAC region HSE manager | Bayer
EPSC : process safety hazards for pharma operation units
Li Liu | EHS manger | BI China EHS&S
Safety maturity model
Barry Bai | Sr. HSE manager | External Manufacturing China Elanco
Road transportation of dangerous goods regulations (JT-617)
Tina Wang | Project director | Dow & Ann Consulting (Shanghai) Ltd.,
How to complete a high-quality equipment containment performance test
Vincent Zhang | IH service leader | Joinhand Consulting Co., Ltd.
IH modelling and its application in quantitative exposure assessmen
Wenjia Xu | Associate Director, Global Safety & Environment | MSD
——————————————————————————————————————
变更管理
邹根平 | 亚太地区HSE经理 | 拜耳
EPSC:制药单元操作中的工艺安全危害及其典型的控制措施
刘立 | EHS 经理 | 勃林格殷格翰
安全成熟度模型
白大明 | HSE高级经理 | 礼来
危险品道路运输规定(JT-617)- 化学品车辆合规性检查
王文 | 项目总监 | 道安咨询(上海)有限公司
如何完成一次高质量的密闭测试
张佳维 | 工业卫生咨询业务负责人 | 章含管理咨询(上海)有限公司
职业卫生模拟及其在定量接触评估中的应用
徐文嘉|副总监,全球安全与环境|默沙东
The first session of the 2022 PSCI China supplier conferences took place virtually on 6 September and covered PiE / AMR and Environment.
Please click “DOWNLOAD” to access to session 1's deck.
The first session includes:
Business continuity plan
Roderick | Technical director | Shanghai STRM Technology Co., Ltd.
PSCI Audit program – practical guidance
Catherine Zhang | Head of HSE experts APAC | PSCI China sub team lead | Bayer
Supplier sharing: CAPR formulation & common audit findings
Ying Lin | EHS & Assistant GM | Zhejiang Raybow Pharmaceutical Co. LTD
Danhua Xu | EHS associate manager | Tianyu Pharmaceutical Co. LTD
The new Data Privacy Law in China
Chris Zhang | Senior partner | Dentons Law Firm
New labor codes
Minnie Mai | Senior technical manager | TUV
——————————————————————————————————————
业务连续性计划
员文权 | 技术总监 | 上海翊员科技有限公司
PSCI审计项目分享
张晓花 |亚太区健康安全环境专家组负责人 | PSCI 中国小组负责人 | 拜耳
供应商实践分享: 不同审计体系共性发现项CAPR制定
林迎 | 总经理助理(分管EHS)| 浙江瑞博制药有限公司
许丹华 | EHS副总经理 | 临海天宇药业有限公司
中国新数据隐私法
张勇 | 高级合伙人 | 大成律师事务所
新劳工法规
麦璐 | 高级技术经理 | 德国莱茵
The second session of the 2022 PSCI China supplier conferences took place virtually on 7 September and covered PiE / AMR and Environment.
Please click “DOWNLOAD” to access to session 2's deck.
The second session includes:
Mass balance work tool
Annie Feng | Principal Consultant | Arcadis
Supplier practice sharing: toxicant minimization in API wastewater
Qinfan Zhang | Director China | Enviolet GmbH
Candy Chen | EHS manager | Zhejiang Hisoar Chuannan Pharma Co., Ltd.
Waste minimization - application of flow chemistry
Jian Tao | Vice general manager | Center of Flow & Continuous Technology (CFCT) | Asymchem
Environmental protection project management
Qingpeng Zhu | Environment protection supervisor | Pharmaron
ESG and sustainability reporting
Ellen Zhang | Senior manager of expert support services, head of China Office | Enhesa
GHG emissions accounting (Supplier Scope 1 & 2)
Zoey Tang | EHS Consultant | Golder
——————————————————————————————————————
2022PSCI线上供应商会议(中国)第二场在9月7日举行,会议内容包括环境中的药物 / 抗生素抗药性,环境。
会议PPT请在下方点击“DOWNLOAD”下载。
第二场会议具体包括:
确定药物生产过程API损失的预测环境浓度
冯婉华博士| 首席顾问 | 凯谛思
供应商实践分享: API废水排放-废水中的毒物减少
张庆凡 | 中国大区总经理 | 恩维勒
陈燕芳 | EHS经理 | 浙江海翔川南药业有限公司
废弃物减少-废弃物减少- 流动化学的应用
陶建 | 副总经理 | 凯莱因医药集团-连续科学技术中心
环保工程建设管理
朱庆鹏 | EHS主管 | 康龙化成(天津)药物制备技术有限公司
ESG与可持续发展报告分享
张赟 | 专家支持服务高级经理,中国办公室负责人 | 音和环保咨询(上海)有限公司
温室气体核算
汤淋麟 | EHS咨询顾问 | WSP Golder
The PSCI is pleased to introduce the Maturity Models in this informative video. The Maturity Models are a very useful tool for suppliers to evaluate their current practices and plot a course for improvement. They allow users to evaluate their company practices against the PSCI Principles and signpost the user to the resources needed to progress.
The PSCI is pleased to introduce the Maturity Models in this informative video. The Maturity Models are a very useful tool for suppliers to evaluate their current practices and plot a course for improvement. They allow users to evaluate their company practices against the PSCI Principles and signpost the user to the resources needed to progress.
This version is subtitled
Today the PSCI published its 2021 Annual Report, summarizing a year of exceptional advancement towards achieving our shared vision of excellence in safety, environmental, and social outcomes across the global pharmaceutical and healthcare supply chain.
Key developments in 2021 include:
Manjit Singh, 2021 PSCI Chair and Associate Director of Corporate Sustainability at Centrient Pharmaceuticals says,
2021 was a year of spectacular growth for the PSCI in membership and activity. Despite the second year of the Covid-19 pandemic, every aspect of the PSCI's program developed and grew from our shared audits to our supplier capability interactions. I have appreciated the professional and energetic contribution of my fellow PSCI members and watched with great pride the growth of the organization and the impact it has upon our entire industry.
You can read about these advancements and more in the report below.
We welcome comments and encourage you to share this report with your networks using the hashtag #PSCIAnnualReport.
The Musi River flows through Hyderabad in Telangana State, Southern India. It is fed by the world-heritage Hussein Sagar lake, also called the “heart of the world”, and used to be enjoyed by locals and tourists alike for its beauty and amenity. Yet, there have been numerous reports of polluted effluents and poor wastewater management practices affecting the lake and the Musi River watershed, impacting local communities over many years.
For World Water Day 2021 (22 March), we published a statement outlining our support for the State government's initiative and our plans to help. We aimed to use our influence to engage with suppliers of PSCI members in Telangana to promote better wastewater practices and encourage positive change. Our aim is that every single supplier with a connection to a PSCI member should be visited, audited or contacted directly to promote the best technologies for wastewater management.
One year later, we're pleased to share an update on progress made. Since March 2021, we have
Our aim is that all suppliers to PSCI members should provide information about their wastewater treatment practices, either via member visit, questionnaire, or a full site audit shared on our platform.
To continue this work, we will
We hope to contribute to restoring the Musi River to its former health.
This document addresses the common questions suppliers have around the PSCI audit program. The key points include:
The Audit Committee has recently conducted a light refresh on the SAQ/Audit Excel template, and the Audit Guidance document. The new versions are now available on the Link.
This webinar covered the following topics:
We were delighted to be joined by:
The recording is available here.
The slides used during the webinar are available for download below.
Our final newsletter of 2021 reflects on our virtual AGM, successful Supplier Conferences, and the launch of our position statement on Human Rights.
At the PSCI, we’re committed to supporting all our members to implement the United Nations’ Guiding Principles on Business and Human Rights (UNGPs) as part of our mission to drive excellence in safety, environmental, and social outcomes across the pharmaceutical and healthcare supply chain.
That’s why for Human Rights Day (10 December), we’re excited to launch our new Position Statement, which provides an overview of the role we play in helping our members and suppliers align their practices with the UNGPs through the PSCI Principles for Responsible Supply Chain Management and our resources.
With our training materials, webinars, and tools we aim not only to ensure the upholding of our Principles, but also to support all our members in implementing the UNGPs across their entire supply chains.
You can read the full statement below.
EXCEL VERSION
PSCI Self Assessment Questionnaire and Audit Report Template for Core Suppliers, External Manufacturers, Component and Material Suppliers
For optimum functionality, we recommend using the most up to date version Microsoft Excel. If you only have access to Excel 2003 or earlier, we recommend using the Word version of the SAQ.
Content Version 6, February 2019
Content Version 7, remote audit options added, October 2020
Content version 7.1, small technical fixes, including correctly referencing finding type on CAP tab, showing suppliers responses to topic areas that are not audited, May 2021
Content version 7.2, fix formating issue of finding classifications on the Company Specific Questions tab, June 2021
Content version 8.0, simplified the Categorisation of Suppliers (Category A, B or C), removed/ added a few questions, rewording some questions, December 2021
Content version 9.0, full review and update of the SAQ. Section names and all questions revised to reflect the updated PSCI Principles, September 2024.
Abbreviated version template for Service Providers & General Manufacturers are also available: Excel version.
The SAQ is currently available in the following languages:
EXCEL VERSION
Abbreviated PSCI Self Assessment Questionnaire & Audit Report Template for Service Providers & General Manufacturers
For optimum functionality, we recommend using the most up to date version Microsoft Excel. If you only have access to Excel 2003 or earlier, we recommend using the Word version of the SAQ.
Content Version 6, February 2019
Content Version 7, remote audit options added, October 2020
Content version 7.1, small technical fixes, May 2021
Content version 7.2, fix formating issue of finding classifications on the Company Specific Questions tab, June 2021
Content version 8.0, simplified the Categorisation of Suppliers (Category A, B or C), removed/ added a few questions, rewording some questions, December 2021
Content version 9.0, full review and update of the SAQ. Section names and all questions revised to reflect the updated PSCI Principles, December 2024.
Full version template for Core Suppliers, External Manufacturers, Component and Material Suppliers are also available and come in multiple languages: Excel version.
The second session of the 2021 PSCI China supplier conferences took place virtually on 10 September and covered PiE / AMR.
The full recording of the session is available here. Slides are available for download below.
2021PSCI线上供应商会议(中国)第二场在9月10日举行,会议内容包括环境中的药物 / 抗生素抗药性。
本次会议的完整视频请点击链接 (提取码 6666) 获取。会议PPT请在下方点击“DOWNLOAD”下载。
The second session includes: 第二场会议具体包括:
This is an explainer video on the PSCI Topic Teams with English subtitles.
This is a short video explaining the Topic Teams of the PSCI.
The fourth session of the 2021 PSCI China supplier conferences took place virtually on 17 September and covered Safety, Process Safety Management (PSM) .
The full recording of the session is available here. Slides are available for download below.
2021PSCI线上供应商会议(中国)第四场在9月17日举行,会议内容包括安全 & 过程安全管理 。
本次会议的完整视频请点击链接 (提取码 txg2 ) 获取。会议PPT请在下方点击“DOWNLOAD”下载。
The fourth session includes: 第四场会议具体包括:
LOTO best practice
Frank Deng, EHS Manager, TÜV Rheinland
上锁挂牌的最佳实践 (50mins 分钟)
邓柱明,EHS经理,德国莱茵
Local government regulations and best practices around hot work and confined space
Shiwei Kong, EHS Head, Zhejiang Langhua Pharmaceutical
动火作业和受限空间作业:各级政府要求与企业最佳实践 (50mins 分钟)
孔识卫, EHS总监, 浙江朗华制药有限公司
Preventive Maintenance for safety equipment
Shuquan Chen, Supplier Operations EHS Manager, Pfizer
安全设备预防性维护 (50mins 分钟)
陈树权,供应商运营EHS经理,辉瑞
The third session of the 2021 PSCI China supplier conferences took place virtually on 16 September and covered Management systems, Industrial hygiene.
The full recording of the session is available here. Slides are available for download below.
2021PSCI线上供应商会议(中国)第三场在9月16日举行,会议内容包括环境中的管理体系 & 工业卫生 。
本次会议的完整视频请点击链接 (提取码 jg38) 获取。会议PPT请在下方点击“DOWNLOAD”下载。
The third session includes: 第三场会议具体包括:
The Practice of Dual-system in Pharmaceutical Enterprise
Guoqiang Peng, EHS Manager Assistant, Hangzhou Zhongmei Huadong Pharmaceutical
双重预防体系(双体系)在制药企业中的运用
彭国强,EHS经理助理,杭州中美华东医药股份有限公司
Pharma wastewater API testing
Congyi Xu, Senior regulatory consultant, CIRS
中国《新化学物质环境管理办法》解析
许丛艺, 资深化学品法规咨询师, 瑞旭集团
Occupational exposure band and control technology in pharmaceutical industry
Chengyin Guo, Deputy-director of EHS, Raybow Pharma
职业卫生分级管控及密闭技术在制药企业中的应用
郭成寅,EHS副总监,瑞博制药
The first session of the 2021 PSCI China supplier conferences took place virtually on 9 September and covered Introduction, Human Rights & Labor, Environment.
The full recording of the session is available here. Slides are available for download below.
2021PSCI线上供应商会议(中国)第一场在9月9日举行,会议内容包括PSCI 最新发展、劳工及环境。
本次会议的完整视频请点击链接 (提取码 9m1p) 获取。会议PPT请在下方点击“DOWNLOAD”下载。
The first session includes: 第一场会议具体包括:
Recent developments within PSCI
Manjit Singh, PSCI Chair, Associate Director - Corporate Sustainability, Centrient
PSCI最新动态
Manjit Singh, PSCI主席,可持续发展副总监,灿盛制药
Audit Committee update: remote audit, audit sharing and supplier self-initiated audit
Kelley Jiang, Head HSE TPRM Operational Excellence Global HSE, Novartis
审核委员会最新内容: 远程审计、共享审计报告与供应商自发PSCI 审核
江戎, 大中国区供应商健康安全环境风险管理主管, 诺华制药
Common findings in PSCI audits under Labor section
Minnie Mai, Senior Technical Manager,TÜV Rheinland
审核劳工部分常见发现项
麦璐, 高级技术经理,德国莱茵
3060 carbon targets and latest government regulations
Stone Huang, lawyer and certified safety engineer, Jin Mao Law Firm 3060双碳目标制定背景双碳目标制定背景及及最新相关法规政策探讨
黄启荣,律师/注册安全工程师, 金茂律师事务所
The issue of Pharmaceuticals in the Environment (PiE) is an increasing public concern. While manufacturing losses represent a small percentage of the overall API mass entering the environment, the PSCI is committed to addressing localized risks that may occur from pharmaceutical manufacturing operations. Many companies are requiring that their facilities – and their major suppliers – establish estimates for API mass loss and the resulting Predicted Environmental Concentration (PEC) in the water body that ultimately receives a facility’s wastewater discharge.
The PSCI-endorsed method for estimating API mass loss is to follow a mass balance approach where the API inputs, outputs, and losses are accounted for, allowing the mass entering wastewater to be estimated. The PSCI has developed a set of detailed guidance documents on how to approach the mass balance calculations, including this presentation which provides an overview of:
We were delighted to be joined on this webinar by:
The recording is available here.
The slides used during the webinar are available for download below.
Participants are invited to share any feedback about this webinar by completing this survey.
The following document provides guidance on potential techniques, methodologies, and available data sources for the calculation of dilution factors. The correct approach to follow is dependent on specific aspects of the risk assessment to be conducted. The PSCI does not advocate a single correct approach or data source, but aims to provide information to help risk assessors design assessments suitable for their requirements.
The contents of this document has been kindly shared by the IAI PiE Task Force.
The following document provides guidance on potential techniques, methodologies, and available data sources for sampling and analysis of pharmaceutical industry wastewater. The correct approach to follow is dependent on specific aspects of the risk assessment to be conducted. The PSCI does not advocate a single correct approach or data source, but aims to provide information to help risk assessors design assessments suitable for their requirements.
The contents of this document has been kindly shared by the IAI PiE Task Force.
Please download to access the agenda for the three-day virtual event.
A clear process has been defined to ensure PSCI Audits are consistent and rigorous. A suite of tools are available to support and guide through the process. Follow the link to read more about:
In order to ensure quality and integrity, PSCI Audits are carried out either by qualified internal auditors working at PSCI member companies or by professional and independent third party audit firms.
PSCI has approved fifteen professional, independent third party audit firms to perform PSCI Audits.
This is an explainer video on the PSCI Governance Structure with English subtitles.
This is a short video explaining the Governance Structure of the PSCI.
Anti-bribery and corruption is one of the issues under the PSCI Principles. The PSCI has commissioned an external benchmark on anti-bribery and corruption (ABC) standards and trends in supply chain. This report has been created to help our member companies and suppliers strengthen the ABC risk management practice in their supply chain.
Background: Regulations around anti‐corruption have been tightened and stakeholder expectations have increased. PSCI wants to encourage and support members and suppliers to continually enhance their anti‐corruption program for compliance with the latest guidelines issued by different regulatory authorities around the globe. Organizations are expected to develop proactive, risk‐based compliance programs that are tailored to the specific risk profile and are reasonably designed to prevent them and their employees/agents/suppliers from engaging in bribery or corrupt acts.
A short explainer video on the PSCI Principles for Responsible Supply Chain Management with English subtitles.
A short explainer video on the PSCI Principles for Responsible Supply Chain Management.
We are very pleased to announce that the 2020 PSCI Annual Report was launched today, summarizing the progress and achievements we have made in advancing responsible supply chain practices within the pharmaceutical and healthcare industries over the past year.
Key developments in 2020 include:
2020 was an extraordinary year, full of large-scale professional challenges for our industry and its supply chain, not to mention the personal challenges we all faced in our own lives. We are proud of the way the PSCI adjusted to these challenges, showing remarkable agility. It is clear that the importance of responsible supply chain practices cannot be overstated and we are pleased to be able to drive positive change across the industry through our collaborative efforts.
We welcome comments and encourage you to share this report with your networks.
Since 2016, a group of PSCI members have been working together to standardize their environmental data request to suppliers through a common set of questions, known as the PSCI Environmental Survey. The benefit is that suppliers should be receiving a common set of questions, but currently companies collect the data using different routes: some use an Excel file, and some a third-party platform.
The PSCI has now built the Environmental Survey into the PSCI platform for suppliers (called The Link). Each of the PSCI’s 50 members will now be able to use that platform to collect data from suppliers and we anticipate that many will do so. We hope that this will streamline and simplify your reporting of environmental and carbon emissions to your customers as one reply can now be shared with many customers.
Watch this webinar to learn more about the new platform and how to use it. You'll hear about:
We were delighted to be joined by Alejandro Fiocco, Partner at Carnstone and Secretariat for the PSCI Environment Team.
The recording is available here. (Please refresh the page if you cannot play the video.)
The slides used during the webinar are available for download below.
Participants (including registrants who were unable to attend) are invited to share feedback about this webinar here.
Please also note that guidance about the Environmental survey is available here (the excel version is accessible here).
More great publicity for the PSCI with thanks to Dan Caldwell (Johnson & Johnson) and Steve Brooks (AMR Alliance) who recently recorded this CPhI podcast on pharmaceuticals in the environment (PiE) and anti-microbial resistance (AMR) and what the industry is doing to help address the challenge.
The PSCI has published a PEC:PNEC calculator tool for manufacturers to use to calculate how to meet safe levels of discharge for active pharmaceutical ingredients (APIs) from their sites.
Image credit: CPhI
This document is a step by step guidance on using the PSCI Environmental survey for suppliers on the PSCI platform. If you have any further questions, please do not hesitate to contact us.
This file contains the latest version of the PSCI Environmental survey for suppliers in Excel. The scoring for each question is detailed and questions are mapped across to the previous version.
Our April newsletter reflects recent activities including our support to the Musi River Revitalization Initiative, the publication of our Benchmarking Survey Insights Report, and our outreach campaign on the vital issue of anti-microbial resistance. It also highlights our upcoming plans and events, such as our Virtual Spring Meeting, and external developments relevant to pharmaceutical supply chains.
At the PSCI, we’re committed to doing everything in our power to limit that the spread and growth of two emerging threats – Pharmaceuticals in the Environment (PiE) and Anti-microbial Resistance (AMR). That’s why for World Health Day, we’re excited today to launch our new Position Statement, which provides a clear explanation of the critical role we play in helping our supplier partners improve their manufacturing practices. The statement has been written to highlight the relevance of the PiE issue along the supply chain, to articulate the PSCI's position on this high profile topic, and to help answer stakeholder inquiries. It includes an overview of all PSCI resources on PiE and AMR-related issues.
Updated “draft” PSCI surface water PEC:PNEC calculator tool
Version: 23 March
Supplier Self-initiated Audit Programme (SSIA) is a pilot programme led by PSCI Audit Committee to encourage suppliers to take a proactive, risk-based approach and self-initiate PSCI audits for their facilities.
With the pilot programme this year, the PSCI will pay for a new Supplier Self-initiated Audit of the supplier facility. The audit should be completed by a PSCI approved audit firm and shared with PSCI members on the Link. Any supplier to PSCI Member can apply to participate in SSIA.
This document for suppliers explains:
Members are welcome to share the brochure with suppliers you would like to see conducting a PSCI audit.
If you are interested in learning more about the programme, please contact Audit Committee Secretariat Blake Zheng for more information. Thank you.
In 2020, we invited the PSCI members to participate in our third formal Member Benchmarking Survey and we are pleased to present the results in this Insights Report. We have used the data collected over the years to share year-on-year trends, as well as provide a snapshot from 2020.
This report paints a picture of rising engagement and increasing focus on responsible procurement. We hope that you find it useful and insightful.
This document provides a guidance for auditors to classify Critical-Major-Minor findings under all five PSCI principles. It is for guidance only and each organization can have its own interpretations.
It is intended to be a living document, which will be reviewed and updated regularly by SAQ/Audit and Audit Guidance sub team.
This document was updated in June 2024 to align with the updated PSCI Principles. For each principle, the examples have been updated and reviewed by a working group formed from the SAQ/Audit and Audit Guidance sub-team. The updated document now also includes complete examples for the minor classification findings.
The second session of the 2020 PSCI Auditor Training (Virtual) took place virtually on 25 November and covered below presentations.
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
The recording of the session is available here. Chinese users can download the recording here (password: PSCI).
Slides are available for download below.
The fourth session of the 2020 PSCI Auditor Training (Virtual) took place virtually on 3 December and covered below presentations.
This session was facilitated by Lei Sun, Partner at Carnstone.
The recording of the session is available here. Chinese users can download the recording here (password: PSCI).
Slides are available for download below.
The third session of the 2020 PSCI Auditor Training (Virtual) took place virtually on 2 December and covered below presentations.
This session was facilitated by Lei Sun, Partner at Carnstone.
The recording of the session is available here. Chinese users can download the recording here (password: PSCI).
Slides are available for download below.
The first session of the 2020 PSCI Auditor Training (Virtual) took place virtually on 24 November and covered below presentations.
PSCI Audit Update 2020
Birgit Isabelle Skuballa, Head HSE Audit & Supplier Management, Bayer
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
The recording of the session is available here. Chinese users can download the recording here (password: PSCI).
Slides are available for download below.
For those interested in becoming PSCI approved audit partners, this document covers:
Last updated December 2020
The PSCI supports the efforts of the State of Telangana to reduce pollution of the Musi River, specifically in the immediate vicinity of the two drainage canals that flow into the world-heritage Hussain Sagar lake where a number of PSCI member suppliers’ operations are situated. Please find our full statement below.
Our winter newsletter reflects recent activities including our Annual General Meeting, supplier conferences, and webinars. It also highlights our upcoming plans, and external developments relevant to pharmaceutical supply chains.
This is a short video on the PSCI Antitrust expectations according to the PSCI Antitrust Policy.
Please refer to the PSCI Antitrust policy, for further information.
The second session of the 2020 PSCI India supplier conferences took place virtually on 29 October and covered Safety, PSM and Industrial Hygiene (IH).
The full recording of the session is available here. Slides are available for download below.
Recordings and slides of individual presentations are also available as individual resources - please click on the relevant links below:
This presentation on Implementing a comprehensive industrial hygiene program – panel presentation was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on Industrial Hygiene Maturity Model was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on Process Safety Management – 2nd supplier case study was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on Process Safety Management – 3rd supplier case study was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on Risk Assessment Keynote was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
The fourth session of the 2020 PSCI China supplier conferences took place virtually on 29 October and covered Safety, PSM and Industrial Hygiene (IH).
The full recording of the session is available here (code:PSCI). Slides are available for download below.
Recordings and slides of individual presentations are also available as individual resources - please click on the relevant links below:
2020 PSCI线上供应商会议(中国)第四场在10月29日举行,会议内容包括安全,过程安全管理和工业卫生。
本次会议的完整视频请点击链接 (提取码 PSCI) 获取。会议PPT请在下方点击“DOWNLOAD”下载。
每个环节单独的视频和PPT也已上传至本网站。请点击下列相关链接获取:
This presentation on PSM Maturity Model was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on Electrical Safety & LOTO was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on Implementing a comprehensive industrial hygiene program – panel presentation was delivered by:
此演讲由下列嘉宾分享如何实施一个全面的工业卫生方案 – 实用三步指南:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on Industrial Hygiene Maturity Model was delivered by:
此演讲由下列嘉宾分享工业卫生成熟度模型:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on PSM – best practice by member was delivered by:
此演讲由下列嘉宾分享工艺安全案例:来自成员公司的最佳实践:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on PSM – supplier case study: MOC’s role in a smoldering incident was delivered by:
此演讲由下列嘉宾分享工艺安全案例:从一起物料的自燃事件看变更管理的重要性:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on Risk Assessment keynote was delivered by:
此演讲由下列嘉宾分享风险评估要旨:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on PSM Maturity Model was delivered by:
此演讲由下列嘉宾分享工艺安全成熟度模型:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on Electrical Safety & LOTO was delivered by:
此演讲由下列嘉宾分享电气安全和挂牌上锁:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on Evolving Ethics Best Practices in the Pharmaceutical Industry: Introduction to PSCI Principles & Antibribery and Corruption was delivered by:
此演讲由下列嘉宾分享在制药行业中深化商业道德最佳实践:介绍PSCI准则、反贿赂和腐败:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on Operationalizing the PSCI Human Rights Principles was delivered by:
此演讲由下列嘉宾分享如何践行PSCI人权准则:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on Evolving Ethics Best Practices in the Pharmaceutical Industry: Introduction to GDPR was delivered by:
此演讲由下列嘉宾分享在制药行业中深化商业道德最佳实践:在制药行业中深化商业道德最佳实践:介绍欧盟《通用数据保护条例》:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
The third session of the 2020 PSCI china supplier conferences took place virtually on 15 October and covered ethics, human rights & labor.
2020 PSCI线上供应商会议(中国)第三场在10月15日举行,会议内容包括商业道德、人权及劳工。
The recording is available here (code:PSCI).
会议视频请点击此 链接 获取 (提取码 PSCI)。
Slides are available for download below.
会议PPT请在下方点击“DOWNLOAD”下载。
Recordings and slides of individual presentations are available as individual resources - please click on the relevant links below:
每个环节单独的视频和PPT也上传至本网站。请点击下列相关链接获取:
OPERATIONALIZING THE PSCI HUMAN RIGHTS PRINCIPLES | 如何践行PSCI人权准则
A CONVERSATION ON FORCED LABOR AND MODERN SLAVERY | 强迫劳工和非自愿劳工
To respect the decision of the speaker, the recording and slides are not shared here.
为尊重嘉宾的意愿,此环节的视频和PPT不在此分享。
The third session of the 2020 PSCI India supplier conferences took place virtually on 15 October and covered Ethics, Human Rights & Labor.
The full recording of the session is available here. Slides are available for download below.
Recordings and slides of individual presentations are also available as individual resources - please click on the relevant links below:
This presentation on Operationalizing the PSCI Human Rights Principles was delivered by:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on A Conversation on Forced Labor and Modern Slavery was delivered by:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on Introduction to PSCI Principles & Antibribery and Corruption was delivered by:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on Introduction to GDPR was delivered by:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on Case studies on WWT technology was delivered by:
此演讲由下列嘉宾分享PiE:废水处理技术案例
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on EWhole effluent toxicity (WET) testing methods and application was delivered by:
此演讲由下列嘉宾分享全废水毒性测试方法与应用:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on How to do a mass balance calculation was delivered by:
此演讲由下列嘉宾分享生态环境中药物活性成分的总量平衡计算 :
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on Environmental hazards of drugs and case analysis was delivered by:
此演讲由下列嘉宾分享药物的环境危害与案例分析 :
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on sustainable packaging was delivered by:
此演讲由下列嘉宾分享可持续包装:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on taking climate action was delivered by:
此演讲由下列嘉宾分享应对环境变化主题:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This is the opening address of the PSCI Virtual China Supplier Conference 2020, delivered by:
此会议内容为PSCI 环境工作小组简介,我们很开心能够邀请两位嘉宾出席:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code:PSCI). The slides are available for download below.
会议视频请点击此 链接 获取 (提取码 PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
The second session of the 2020 PSCI China supplier conferences took place virtually on 24 September and covered Environment, Pharmaceuticals in the Environment/AMR.
The full recording of the session is available here (code:PSCI). Slides are available for download below.
Recordings and slides of individual presentations are also available as individual resources - please click on the relevant links below:
2020 PSCI线上供应商会议(中国)第二场在9月24日举行,会议内容包括环境,环境中药物残留及抗微生物药物耐药性。
本次会议的完整视频请点击链接 获取 (提取码 PSCI) 获取会议视频。会议PPT请在下方点击“DOWNLOAD”下载。
每个环节单独的视频和PPT也已上传至本网站。请点击下列相关链接获取:
The first session of the 2020 PSCI China supplier conferences took place virtually on 17 September and covered PSCI Updates and Management Systems.
The full recording of the session is available here (code: PSCI). Slides are available for download below.
Recordings and slides of individual presentations are also available as individual resources - please click on the relevant links below:
2020 PSCI线上供应商会议(中国)第一场PSCI最新发展和管理体系的完整视频在此链接 获取。 (提取码 PSCI)。会议PPT请点击下方“DOWNLOAD”下载。
每个环节单独的视频和PPT也已上传至本网站。请点击下列相关链接获取:
This presentation on PSCI capability building vision and plans was delivered by:
此演讲由下列嘉宾分享PSCI 供应商能力建设愿景和规划:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code: PSCI). The slides are available for download below.
会议视频请点击此 链接 (提取码:PSCI) 获取会议视频。会议PPT请在下方点击“DOWNLOAD”下载。
This is the opening address of the PSCI Virtual China Supplier Conference 2020, delivered by:
此会议内容为PSCI和本地团队欢迎所有会议参议者,我们很开心能够邀请两位嘉宾出席:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code: PSCI). The slides are available for download below.
会议视频请点击此 链接获取(提取码:PSCI) 。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on the future of online learning was delivered by:
此演讲由下列嘉宾分享线上学习主题:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code: PSCI). The slides are available for download below.
会议视频请点击此链接 (提取码:PSCI) 获取。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation detailing our audit finding analysis was delivered by:
此演讲由下列嘉宾分享PSCI 共享审计发现研究:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code: PSCI). The slides are available for download below.
会议视频请点击此链接 获取(提取码:PSCI)。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation introducing Pharmaron’s work arrangement, prevention and control organization, and normalized control in the fight against COVID-19 was delivered by:
此演讲由下列嘉宾分享康龙化成的新冠疫情防控经验:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code: PSCI). The slides are available for download below.
会议视频请点击此链接获取(提取码:PSCI) 。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation sharing the key points from China’s “Measures for Environmental Management Registration of New Chemical Substances” was delivered by:
此演讲由下列嘉宾介绍中国新化学物质环境管理办法的最新进展:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code: PSCI). The slides are available for download below.
会议视频请点击此链接 获取 (提取码:PSCI) 获取会议视频。会议PPT请在下方点击“DOWNLOAD”下载。
This presentation on PSCI Management System Maturity Model was delivered by:
此演讲由下列嘉宾分享PSCI 管理系统成熟度模型:
This session was facilitated by Maggie Zhang, Partner Manager at Carnstone.
本环节由凯嵘管理咨询合伙人经理张缤莉主持。
The recording is available here (code: PSCI). The slides are available for download below.
会议视频请点击此链接获取 (提取码:PSCI) 。会议PPT请在下方点击“DOWNLOAD”下载。
The primary focus of this document is to provide a consistent guidance for pharmaceutical companies to calculate GHG emissions in their upstream and downstream value chains. It provides methodologies consistent with recommendations from the GHG Protocol for calculating emissions which are tailored for each different category.
This document was developed by the Pharmaceutical Environment Group (PEG) and its participating companies, who have kindly shared it with the PSCI for diffusion.
This presentation on the future of online learning was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on PSCI Management System Maturity Model was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on Indian Case Study on Controlling API Releases Including Wastewater Treatment/Analysis was delivered by:
This session was facilitated by Annabel Buchan, Partner Manager at Carnstone.
The recording is available here. The slides are available for download below.
This introduction to sampling and analysing APIs in wastewater was delivered by:
This session was facilitated by Annabel Buchan, Partner Manager at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on Draft Indian Wastewater Standards - Current Status and Possible Ramifications for Indian companies was delivered by:
This session was facilitated by Annabel Buchan, Partner Manager at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on Sustainable Packaging was delivered by:
This session was facilitated by Annabel Buchan, Partner Manager at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on Taking Climate Action was delivered by:
This session was facilitated by Annabel Buchan, Partner Manager at Carnstone.
The recording is available here. The slides are available for download below.
This PSCI Environment Sub-Team Overview was delivered by:
This session was facilitated by Annabel Buchan, Partner Manager at Carnstone.
The recording is available here. The slides are available for download below.
The second session of the 2020 PSCI India supplier conferences took place virtually on 24 September and covered Environment and Pharmaceuticals in the Environment/AMR.
The full recording of the session is available here. Slides are available for download below.
Recordings and slides of individual presentations are also available as individual resources - please click on the relevant links below:
This presentation on Safety Culture was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on India Chemical Management & Safety Rules (ICMSR) was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on learnings from responding to Covid-19, an experience sharing from Pfizer, India was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
This presentation detailing our audit finding analysis was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
This presentation on PSCI capability building vision and plans was delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
This is the opening address of PSCI Virtual India Supplier Conference 2020, delivered by:
This session was facilitated by Simon Hodgson, Managing Partner at Carnstone.
The recording is available here. The slides are available for download below.
The first session of the 2020 PSCI India supplier conferences took place virtually on 17 September and covered PSCI Updates and Management Systems.
The full recording of the session is available here. Slides are available for download below.
Recordings and slides of individual presentations are also available as individual resources - please click on the relevant links below:
The webinar explored best practice wastewater treatment methods for managing the release of APIs into the environment from pharmaceutical manufacturing sites. It was delivered by renowned experts and featured case studies on novel treatment technologies.
We were delighted to be joined by the following speakers:
Participants are invited to share feedback about this webinar here.
The recording is available here. (Please refresh the page if you cannot play the video.)
The slides used during the webinar are available to download below.
Please also note that registration for the second session of our supplier conferences is open until 17 September. Topics covered will include climate change (GHG) & sustainable packaging ; sampling and analysing APIs in wastewater with case studies ; a case study on Controlling API Releases; environmental hazards of drugs and case analysis ; and mass balance calculations. To register, please click here for the China session and here for the India session.
Finally, we want your views! We're encouraging all suppliers to take this short survey to help the PSCI understand the impact it has on suppliers.
The agenda for China conference is updated on 10 September 2020. Please select the language that you prefer to use before downloading it.
下载会议议程前请先选择语言:英文、简体中文。
This draft agenda for India conference is updated on 9 September 2020.
A PSCI Sub-Team is seeking a consultant to undertake approximately 10 days’ research looking at standards, trends and company practices to prevent bribery and corruption in supply chains. The work will be carried out in August and September, and would suit a small consultancy or single individual with the appropriate expertise. All of the details are in the attached RFP document. Proposals are due by 31st July.
Proposals are to be sent to the PSCI email address: info@PSCInitiative.org.
The PSCI has recently published its Summer newsletter, reflecting on achievements in the first half of 2020 and looking ahead at a busy programme of exciting events and projects for the rest of the year.
Download the newsletter below to read about our efforts to digitalize our offer to suppliers, our position on important issues such as PiE/AMR, and the partnerships we’re forming with Indian and Chinese organizations. You’ll also find updates on PSCI audit findings from the past three years, our take on emerging issues in supply chains – and more!
This document is for members to use when promoting the PSCI. This version is the A4 document for in-house printing, however US paper size and commercial printing documents are available upon request.
The Chinese (Simplified) version of the pamphlet was published in April 2019.
To request alternative versions, please contact the PSCI Secretariat.
Last updated December 2021
This webinar first aired on 27 May and explained the updates and changes to the Audit Guidance document. We were delighted to be joined by the following speaker:
The webinar attracted over 100 registrants and around 90 people joined on the day.
Participants are invited to share feedback about this webinar here.
The recording is available here.
The slides used during the webinar are available to download below.
Finally, we want your views! We're encouraging all suppliers to take this short survey to help the PSCI understand the impact it has on suppliers.
We are very pleased to announce that the PSCI 2019 Annual Report is now available, summarising our collective efforts towards responsible supply chains during 2019.
Key developments in 2019 include:
In 2020, much of business will be operating in a changed environment. It remains to be seen exactly how this will affect the industry and our supply chain practices. Our focus on efficient sharing of audits and virtually-enabled capability building activities is even more important in the wake of Covid-19. Whatever changes arise from this crisis, responsible supply chain practices will be central to our industry’s response.
We welcome comments and encourage you to share this report with your networks.
We are proud to launch our online platform specially-developed for the supplier community. The Link is a place for suppliers to connect, engage and to advance their responsible supply chain practices. The attached announcement provides an overview of the platform and details on how to get started.
You can click here to register.
This announcement provides more information about our supplier community platform, providing more information on why suppliers should engage.
On behalf of the Board and Communications Committee and with thanks to the Pharmaceuticals in the Environment (PiE)/ Anti-Microbial Resistance (AMR) sub-team for their expert input, we are sharing the recently adopted PSCI Position Statement on PiE & AMR. It has been written to highlight the relevance of the PiE issue along the supply chain, to articulate PSCI's position on this high profile topic, and to help answer stakeholder inquiries. It includes an overview of all PSCI resources on PiE and AMR-related issues.
This document can be used by suppliers to document agreed corrective actions, the type of finding(e.g. Critical-Other, Critical-Major/Minor), the time period for completion, the type of verification (e.g. desk top review of documents or follow up visit), the responsible individual at the supplier for the individual corrective actions, details on the implemented corrective action and a status assignment (open/closed).
Since 20 April 2022, the Word versions of the templates (Full, Abbreviated, CAPR) have been removed completely following a consultation process and notice period. Access to these is only be possible in exceptional circumstances and by contacting the PSCI Secretariat. Please use the Excel versions: Full, Abbreviated, CAPR
This document can be used by suppliers to document agreed corrective actions, the type of finding(e.g. Critical/Major/Minor), the time period for completion, the type of verification (e.g. desk top review of documents or follow up visit), the responsible individual at the supplier for the individual corrective actions, details on the implemented corrective action and a status assignment (open/closed).
Content version v4.0, March 2020
Content version v5.0, December 2021
Supplier Data Sharing Agreement (Last updated March 2024).
A Chinese version is now available.
Our latest newsletter, accessible at the link below, reflects recent activities including our Annual General Meeting, supplier conferences and webinars. It also highlights our upcoming plans, and external developments relevant to pharmaceutical supply chains.
Please find the global dial-in numbers in the attached PDF.
We are looking forward to speaking with you.
Presentation Pack from the 2019 PSCI Auditor Training, 26-27 September in Hangzhou, China.
You can also find the recap video here.
Please find the links below to the training slides of both days.
This webinar initially aired on Thursday 24th October 2019.
In this webinar we explored what is meant by modern slavery, where it is found in the world and how it can be addressed in the pharma supply chain.
Our speaker covered the evolving legislative landscape for modern slavery as it relates to pharma, how to identify risks and case study examples of how to address them.
We were delighted to be joined by the following speaker:
You can access the slide deck here.
Graphic of PSCI SDG mapping. We recently mapped PSCI Principles and activities against the UN's Sustainable Development Goals. It was an interesting exercise, revealing where our missions align. We plan to build on this by applying the SDG framework in ongoing and future workstreams.
Click below to download the full graphic:
Introduction presentation for PSCI audit opening meeting.
Version 1 - 07/02/2017
Version 2 - 01/08/2019
Version 3 - 15/04/2020
Version 4 - 31/08/2020
Version 5 - 31/08/2021
Version 6 - 20/12/2021
Agenda for the 2019 Supplier Conference held in Hyderabad, India.
This webinar initially aired on 18th July 2019
In this webinar we will be looking at the impact of pharmaceutical manufacturing on antimicrobial resistance, as well as demonstrating PSCI's PEC:PNEC calculator tool.
Our speakers will be covering the issues associated with antimicrobial drugs in wastewater, targets for environmental risk assessment of antimicrobial drugs, and how to assess the risk of antimicrobial drugs in wastewater.
We are delighted to be joined by the following speakers:
Agenda for the 2019 Supplier Conference held in Hangzhou, China.
This presentation summarises who we are as PSCI and includes an overview of:
Latest version: January 2026
This document gives a summary of the PSCI Audit Program and process and answers frequently asked questions including:
Last updated November 2025
This template was produced by Aaron Duff, Director of EHS&S at Johnson & Johnson, and was showcased during the PSCI Webinar: Emergency Preparedness and Response on 27th June 2019.
This template was produced by Aaron Duff, Director of EHS&S at Johnson & Johnson, and was showcased during the PSCI Webinar: Emergency Preparedness and Response on 27th June 2019.
This webinar was initially aired on 27th June 2019
The webinar will give an overview of the questions relating to emergency preparedness & response in the PSCI audit questionnaire and explain the PSCI's expectations.
The webinar will focus on how to prepare a good site emergency response plan that covers the minimum requirements. You will also hear about the basic elements that should be considered when performing an impact assessment of neighboring industries on your site & vice versa.
We are delighted to be joined by the following speaker:
Aaron Duff, Director of EHS&S at Johnson & Johnson
Presentation Pack from the 2018 PSCI Auditor Training, 4 - 5 December in Vienna, Austria
This maturity model has been developed:
Over the past year the PSCI has gone from strength to strength, growing both in numbers and influence. In line with our growing reach, we have produced a short video explaining the work of the Initiative. We hope that members and stakeholders share this with their networks, and that it helps those unfamiliar with our work understand what we do.
The film is now also available with subtitles in the following languages, click on the links to view the videos on Vimeo:
"2018 has seen PSCI gain more members, reach more suppliers, perform more audits and host more events than ever before. The growth in our membership is a huge achievement but it is the growth in our impact that I am most proud of.
In the pages of this report you will read about our other achievements this year: holding members accountable, setting up a Board Advisory Panel, sharing data on suppliers’ carbon emissions, and a new visual brand identity and logo. Perhaps the most important pages are the ones that show our impact; the suppliers trained, the audits completed and followed up, the downloads of advisory documents from our technical library."
Dr Birgit Skuballa, Bayer (Chair of the PSCI during 2018)
This is a recording of the webinar on ways to assess and manage “Water Stress” inside your company and in your supply chain.
Please find a link to the webinar on the latest SAQ & Audit Report Template Update.
WORD VERSION
Abbreviated PSCI Self Assessment Questionnaire & Audit Report Template for Service Providers & General Manufacturers
Version 5, February 2019
Version 6, remote audit options added, October 2020
Since 20 April 2022, the Word versions of the templates (Full, Abbreviated, CAPR) have been removed completely following a consultation process and notice period. Access to these is only be possible in exceptional circumstances and by contacting the PSCI Secretariat. Please use the Excel versions: Full, Abbreviated, CAPR
This PSCI Audit Sharing Platform User Guide explains how to use the Audit Sharing Platform for members. The platform underpins the PSCI Audit Sharing Program. It is the platform for sharing audits with the PSCI membership.
Please refer to the PSCI Shared Audit Program Guidance for more information about the PSCI Audit Sharing Program.
This document is designed to be used by PSCI members, audit contractors and suppliers. It provides a detailed overview of the audit process and corresponding roles and responsibilities at each stage of the process.
Version 8, November 2025
Updated to align with the updated PSCI Principles and Strategy, inclusion of the digital Data Sharing via the PSCI Audit Platform, updated audit uploading requirements for full members, introduced online SAQ and removed reference of word version template, referenced the Audit Checklist as a separate resource in Chapter 10 and added section on Managing Audit Related Issues in the appendix.
This PSCI webinar is a training video for auditors. This was recorded in September 2018.
The slides shown in this webinar can be found here.
Day 2 presentation pack from the 2018 Supplier Conference in Shanghai, China.
Day 1 presentation pack from the 2018 Supplier Conference in Shanghai, China.
This is the agenda for 2018 PSCI India Supplier Conference.
This is recording of the PSCI sponsored webinar on evaluating supplier ethics and compliance practices and programs which took place on 25th July 2018.
This is the agenda for 2018 PSCI China Supplier Conference.
This is the slide deck from the PSCI sponsored webinar on how to manage APIs in manufacturing effluent (Part 4) which took place on 10th July 2018.
The webinar gave a brief refresher on Pharmaceuticals in the Environment (PiE), Anti-Microbial Resistance (AMR) and Predicted No-Effect Concentration (PNEC) first principles, and introduced a PSCI resource page where PNECs can be found. It also included step-by-step guidance on how to locate PNECs and use them.
The webinar recording is available here.
This is recording of the PSCI sponsored webinar on how to manage APIs in manufacturing effluent (Part 4) which took place on 10th July 2018.
The webinar gave a brief refresher on Pharmaceuticals in the Environment (PiE), Anti-Microbial Resistance (AMR) and Predicted No-Effect Concentration (PNEC) first principles, and introduced a PSCI resource page where PNECs can be found. It also included step-by-step guidance on how to locate PNECs and use them.
The webinar slide deck is available here.
This document defines the PSCI member audit uploading requirement.
Refreshed in May 2025 to align with the audit uploading expectations in the updated PSCI Strategy 2024 - 2026.
The following are useful resources for obtaining PNEC values.
Inside the report, you will find highlights and achievements from 2017, a busy and important year for PSCI, and our plans for 2018.
This is a short summary of the new strategy, and the process taken to achieve it.
Presentation Pack from the 2017 PSCI Auditor Training, 20 - 22 November, in Shanghai, China.
Presentation Pack from the 2017 PSCI Auditor Training, 20 - 22 November, in Shanghai, China.
Presentation Pack from the 2017 PSCI Auditor Training, 20 - 22 November, in Shanghai, China.
This Excel template is designed to help PSCI members, suppliers and audit firms check auditors against the PSCI's requirements, to ensure that they have the expertise needed to conduct PSCI audits. It is also used by new audit firms in their application process to demonstrate their capacity.
This evaluation tool is also known as PSCI Auditor Information Template (HSE & Social Auditors).
Last updated March 2021.
Agenda for 2017 Supplier Conference in Hyderabad, India
Green Chemistry Pharma Week Photo Report February 2017
Presentation booklet from the 2017 PSCI Auditor Training, 28 Feb - 1 March, in Hyderabad, India
This is the slide deck for the PSCI sponsored webinar on how to manage APIs in manufacturing effluent (Part 3) which took place on 25th October 2016. The webinar looked at advanced technologies to reduce API loss with guest speakers from the Temple University WET Centre and AECOM.
This is recording of the PSCI sponsored webinar on how to manage APIs in manufacturing effluent (Part 2) which took place on 15th June 2016. The webinar provided step-by-step guidance on this ‘spotlight’ issue for our industry and covered the following topics:
The webinar's slide-deck is available here.
This is the slide deck for the PSCI sponsored webinar on how to manage APIs in manufacturing effluent (Part 2) which took place on 15th June 2016. The webinar provided step-by-step guidance on this ‘spotlight’ issue for our industry and covered the following topics:
The webinar recording is available here.
This is the slide deck for the PSCI sponsored webinar on how to manage APIs in manufacturing effluent which took place on 27th January 2016. The webinar provided step-by-step guidance on this ‘spotlight’ issue for our industry and covered the following topics:
The webinar recording is available here.
This is a recording of the PSCI sponsored webinar on how to manage APIs in manufacturing effluent which took place on 27th January 2016. The webinar provided step-by-step guidance on this ‘spotlight’ issue for our industry and covered the following topics:
The webinar slide-deck is available here.
This PSCI Audit Sharing Platform User Guide explains how to use the Audit Sharing Platform for suppliers. The platform underpins the PSCI Audit Sharing Program. It is the platform for sharing audits with the PSCI membership.
Please refer to the PSCI Shared Audit Program Guidance for more information about the PSCI Audit Sharing Program.
Abstract: This Caldwell et al. study describes guidance intended to assist pharmaceutical manufacturers in assessing, mitigating, and managing the potential environmental impacts of active pharmaceutical ingredients (APIs) in wastewater from manufacturing operations, including those from external suppliers.
This document explains the PSCI membership fees structure and the Supplier Partnership fee.
New version added: October 2024
PowerPoint on the fundamentals of industrial hygiene.
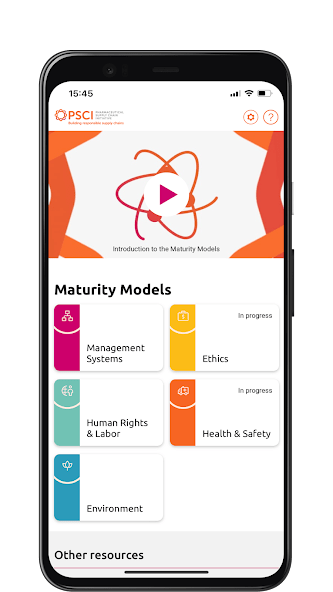
The PSCI Maturity App provides an interactive experience of the PSCI Maturity Models. Available on Android and iOS.